GST : 24AAJCA7955Q1ZW
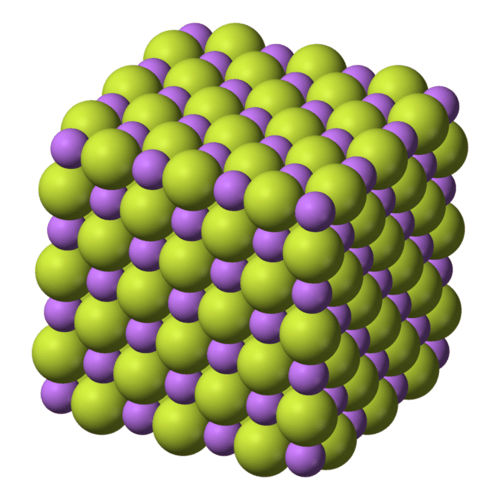
Lithium Fluoride
Product Details:
- Melting Point 845 C (1,553 F; 1,118 K)
- CAS No 7789-43-7
- Product Type Lithium Fluoride
- Purity 98%
- Grade Industrial
- Storage Room Temperature
- Application Industrial
- Click to view more
X
Lithium Fluoride Price And Quantity
Lithium Fluoride Product Specifications
- Lithium Fluoride
- 845 C (1,553 F; 1,118 K)
- Industrial
- 98%
- 7789-43-7
- Industrial
- Room Temperature
Lithium Fluoride Trade Information
- 50 Per Month
- 2-12 Week
Product Description
We have marked a distinct position in the market by offering superior quality of Lithium Fluoride that is utilized as a flux in the production of ceramics such as enamels glasses and glazes Formulated as per the international quality standards this chemical also finds usage in brazing and welding fluxes and molten salt chemistry in metallurgy As per customers requirements our Lithium Fluoride chemical compound is available in various customized packaging options The offered product is tested on various parameters in order to ensure its purity.
Lithium Fluoride Properties:
1. CAS Number: 7789-24-4
2. Chemical formula: LiF
3. Molar mass: 25.939(2) g/mol
4. Appearance: White powder or colorless hygroscopic crystals
5. Density: 2.635 g/cm3
6. Melting point: 845 degree centigrade
7. Boiling point: 1,676 degree centigrade
Lithium Fluoride Applications:
1. Nuclear Industry: Lithium fluoride is used in nuclear reactors as a neutron absorber and as a solid-state thermoluminescent dosimeter (TLD) to measure exposure to ionizing radiation. It is commonly employed in radiation monitoring devices due to its ability to capture and store radiation energy, which can then be measured.
2. Optics: LiF is transparent to ultraviolet (UV) wavelengths and has a high refractive index, making it useful in optical components such as lenses, prisms, and windows for UV applications. It is also used in the production of excimer lasers, which are used in semiconductor manufacturing, medical applications, and scientific research.
3. Electronics: Lithium fluoride is utilized as a flux in the production of ceramic capacitors and in the manufacture of specialized glasses for radiation detection in nuclear physics experiments. It is also employed in the synthesis of lithium-ion batteries, which are widely used in portable electronics and electric vehicles.
4. Chemical Industry: LiF is sometimes used as a catalyst in certain organic synthesis reactions due to its Lewis acid properties. It can also be found in the production of specialty chemicals and pharmaceuticals.
5. Thermoluminescent Dosimetry: In medical dosimetry, lithium fluoride is utilized in thermoluminescent dosimeters (TLDs) to measure radiation doses received by patients during radiation therapy. These dosimeters are sensitive to ionizing radiation and can provide accurate measurements of radiation exposure.
6. Alloys and Metallurgy: Lithium fluoride can be used as a flux in metallurgical processes, particularly in the production of aluminum and magnesium alloys. It helps to remove impurities and improve the quality of the final metal products.
7. Research and Development: LiF is frequently employed in research laboratories for various experimental purposes, including as a substrate for thin film deposition techniques such as molecular beam epitaxy (MBE) and physical vapor deposition (PVD). It is also used in spectroscopy studies due to its optical properties.
Lithium Fluoride FAQ:
Q. Is lithium fluoride toxic?
Ans: Lithium fluoride is generally considered to have low toxicity. However, as with handling any chemical, precautions should be taken to avoid ingestion, inhalation, or contact with skin and eyes. It's important to follow safety guidelines and use appropriate protective equipment when working with LiF.
Q. How is lithium fluoride produced?
Ans: Lithium fluoride can be produced through the reaction of lithium carbonate or lithium hydroxide with hydrofluoric acid. Another method involves reacting lithium metal with fluorine gas.
Q. Can lithium fluoride be recycled?
Ans: Yes, lithium fluoride can be recycled through various methods, such as recovery from waste streams in industrial processes or through specialized recycling techniques for specific applications like lithium-ion batteries.
Q. Is lithium fluoride used in medicine?
Ans: Yes, lithium fluoride is used in medical dosimetry to measure radiation doses received by patients during radiation therapy. It is incorporated into thermoluminescent dosimeters for this purpose.
Q. Does lithium fluoride have any other special properties?
Ans: Apart from its optical and radiation-related properties, lithium fluoride has high thermal stability and is chemically inert under normal conditions. It also exhibits piezoelectric properties, making it useful in certain electronic applications.
Applications of Lithium Fluoride in Industry
Lithium fluoride is widely used in industrial settings due to its robustness and properties such as high melting point and purity. It is effective for manufacturing specialized glasses, flux materials, and as a coating in optics.
Product Properties and Usage
This material exhibits exceptional stability and high purity. These qualities make it an excellent candidate for specialized industrial uses. It is also securely stored at room temperature to ensure its long-lasting effectiveness.
FAQs of Lithium Fluoride:
Q: What are the typical applications of lithium fluoride in industry?
A: Lithium fluoride is widely utilized in industrial applications such as in the production of specialized glasses, as a flux in aluminum manufacture, and as a component in lithium-ion battery production.Q: How should lithium fluoride be stored?
A: Lithium fluoride should be stored in a dry environment at room temperature to ensure its stability and to prevent contamination by moisture.Q: When is lithium fluoride used as a flux material?
A: Lithium fluoride is commonly used as a flux material during the smelting of aluminum and other metals to help lower the melting point of the raw materials.Q: Where is lithium fluoride commonly utilized?
A: It is primarily used in industrial settings like glass manufacturing, nuclear applications, and as coatings for optics.Q: What process makes use of lithium fluoride in the nuclear industry?
A: In the nuclear industry, lithium fluoride is used in molten salt reactors where it acts as a component of the molten salt mixture due to its high melting point and stability under extreme conditions.Q: How does the purity of lithium fluoride affect its usage?
A: The high purity level, such as 98%, ensures that lithium fluoride can be reliably used in precision applications requiring minimal contamination, such as in optics and energy solutions.Q: What are the benefits of using lithium fluoride for glass manufacturing?
A: In glass manufacturing, lithium fluoride aids in improving the thermal and mechanical properties of specialty glasses, such as those used in optical equipment.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Lithium' category
Back to top






 Call Me Free
Call Me Free
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese